Welcome! You might have scanned the QR code or have simply wandered around Entire Ocean to reach the front row of my first ever conferencing at the IX International Symposium of Marine Sciences (10-12 July 2024, Valencia, Spain).
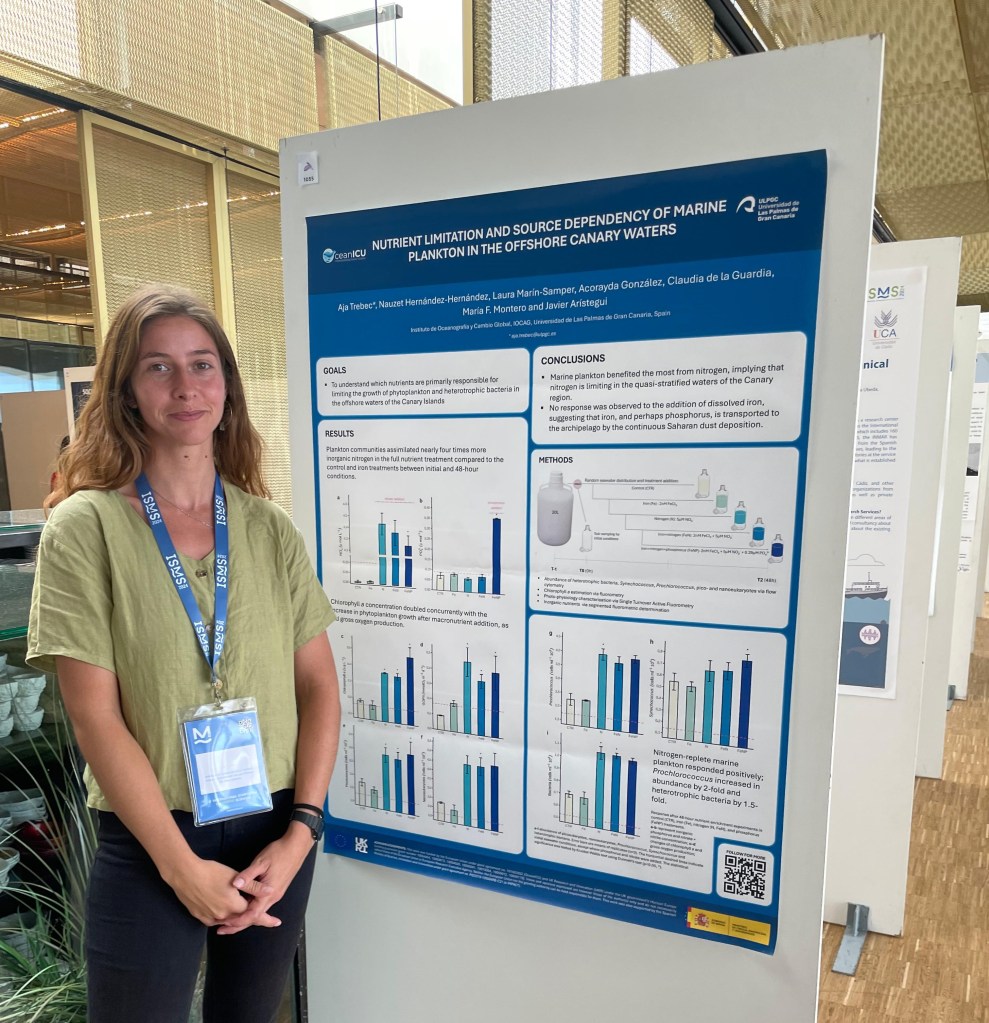
As we rush between the posters, chance is you wanted to get a thorough insight into my work in progress on “Nutrient limitation and source dependency of marine plankton in the offshore Canary waters”. Here, I provide you with the digital version of the poster and extra details of this study.
BACKGROUND AND GOALS: The Canary archipelago is an oligotrophic region, exposed to late winter mixing, mesoscale upwelling of deep oceanic waters and dust deposition from the neighbouring Sahara Desert. These processes could alleviate nutrient limitation by delivering macro- and micronutrients essential for the growth of marine plankton, such as nitrogen, phosphorus and iron. Our goal in this study was to understand which nutrients are primary limiters of phytoplankton and heterotrophic bacterial growth in the offshore waters of the Canary Islands.
METHODS: Offshore seawater was collected on the 22nd of August of 2023 to perform the nutrient enrichment bioassay experiment. Seawater was distributed into transparent polycarbonate Nalgene bottles and spiked with iron, nitrate and phosphorus: Fe: 2nM FeCl3; N: 5μM NO3–; FeN: 2nM FeCl3 + 5μM NO3–; FeNP: 2nM FeCl3 + 5μM NO3– + 0.29μM PO43-. Samples were collected for initial conditions and after 48-hour incubation under irradiance and temperature regimes mimicking natural conditions. Marine plankton abundance of bacteria, Synechococcus, Prochlorococcus, pico- and nanoeukaryotes were estimated via flow cytometry with CytoFLEX and CytoSense instruments. Larger functional groups, such as diatoms, dinoflagellates and ciliates, were analyzed using flow imaging with the FlowCAM (unpublished). Chlorophyll a concentration was acquired via fluorometry, while photo-physiology was characterized via Single Turnover Active Fluorometry with the LabSTAF. Inorganic nutrients, such as oxidized nitrogen species, phosphate and silicate were measured via segmented fluorometry using a SEAL Analytical AutoAnalyzer3.
ACKNOWLEDGEMENTS: I thank every person of the Biological Oceanography Group for great advice to navigate through the first steps of a doctoral career, and motivation to keep sailing forward.
RELATED REFERENCES:
- Capone, D. G., & Hutchins, D. A. (2013). Microbial biogeochemistry of coastal upwelling regimes in a changing ocean. Nature Geoscience, 6(9), 711-717.
- Fu, W., Randerson, J. T., & Moore, J. K. (2016). Climate change impacts on net primary production (NPP) and export production (EP) regulated by increasing stratification and phytoplankton community structure in the CMIP5 models. Biogeosciences, 13(18), 5151-5170.
- López-García, P., Gelado-Caballero, M. D., Patey, M. D., & Hernández-Brito, J. J. (2021). Atmospheric fluxes of soluble nutrients and Fe: More than three years of wet and dry deposition measurements at Gran Canaria (Canary Islands). Atmospheric Environment, 246, 118090.
- McNair, H. M., Brzezinski, M. A., & Krause, J. W. (2018). Diatom populations in an upwelling environment decrease silica content to avoid growth limitation. Environmental microbiology, 20(11), 4184-4193.
- Yuan, Z., Achterberg, E. P., Engel, A., Wen, Z., Zhou, L., Zhu, X., Dai, M. and Browning, T. J. (2023). Phytoplankton community response to episodic wet and dry aerosol deposition in the subtropical North Atlantic. Limnology and Oceanography, 68(9), 2126-2140.

